A Year in Review 2025
2025 has been a transformational year for Unravel Biosciences, marking its first year as a clinical-stage therapeutics company.
Welcome to our Year in Review.
A Message from the CEO
RICHARD’s Letter
Richard Novak, phdCEO & Co-FounderA Snapshot of Our Progress
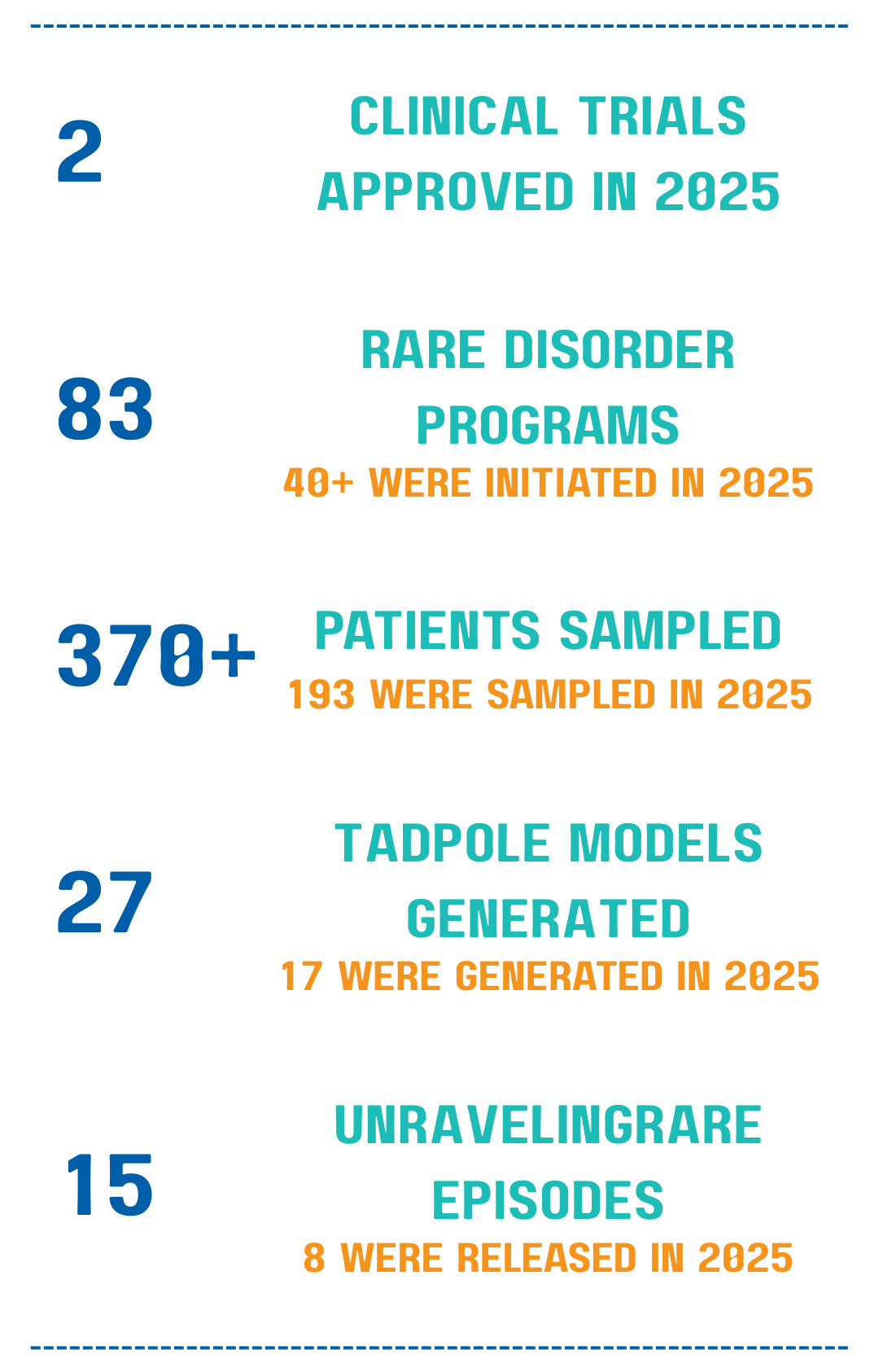
It’s been a busy year for Unravel. Here’s a quick count of some of our work.
Changing the Paradigm of Drug Development: The RVL-001 Clinical Trials
The recent approvals of our RVL-001 clinical trials for Rett syndrome and Pitt Hopkins syndrome have been a major milestone of 2025, with first dosing anticipated in January 2026.
Using our Predictable Medicine™, which combines our datamine of real patient data and our computational AI platform, BioNAV™, we create Living Molecular Twins (LMT) to resemble real patients living with rare disorders. These LMTs enable us to reach the clinic at an accelerated pace by allowing us to understand how real patients will respond to a therapeutic before it ever enters the clinic. We discovered RVL-001 for Rett syndrome in 2019 and Pitt Hopkins syndrome in 2023. With significant gaps in development for financing and starting Unravel, only 2.5 years were needed from discovery to phase I approval.
The clinical trials will be conducted at the Universidad de Antioquia’s Center for Technological Development (“PECET”) in Medellin, Colombia, with Dr. Carolina Lesmes as the Principal Investigator. Thank you to Dr. Lesmes and the PECET team for their hard work and dedication.
Moving into the new year, Unravel will continue pursuing molecules for patient populations with high unmet need using Predictable Medicine™ and our LMTs.
rareSHIFT™
Unravel currently has 83 rare disorder programs. Each gene can be seen to the left, along with the number of patients.
Reaching Across Disorders
*hover your mouse over the dots to see the number of patients we work with from each location*
rareSHIFT™ utilizes a non-invasive sampling modality that allows us to work with patients all over the world. Check out all of the places that we’ve been privileged enough to work with patients thus far.
Working Across the World
*hover your mouse over the genes to see the number of patients we work with from each disorder*
New Partnerships
In 2025, we partnered with many patients, families, and foundations through rareSHIFT™. This year, we partnered with 18 new foundations.
rareSHIFT™ studies entail making Living Molecular Twins of patient populations based on real patient data. We help families and foundations accelerate their therapeutic odysseys, predicting which existing therapeutics will be the most efficacious, while also indicating which mechanisms should be targeted. Starting in patients enables understanding of how a patient population will respond prior to extensive research into a single drug target that does not have guaranteed success.
Our work with patient populations identifies deep insights about patient populations, often identifying multiple subgroups within a population that respond to therapeutics similarly. Not only do these studies identify the minimum number of molecules needed to treat the maximum number of patients, but they also pave the way for us to defragment rare disease, looking for similar drug responders not just within a disorder but across various disorders, creating larger groups of therapeutic responders to create commercially viable markets for molecules.
In addition, we began a collaboration with COMBINEDBrain to enable large-scale drug discovery for rare neurological disorders. This collaboration will catalyze our goal of defragmenting rare disease. As a consortium for rare neurological disorder advocacy groups, COMBINEDBrain will accelerate its members’ efforts to identify drug candidates and therapeutic mechanisms through the rareSHIFT™ program. Thus, Unravel can identify shared mechanisms to further syndicate our drug development approach and defragment rare disease.
We are grateful for the financial support of all our partners.
Diverse Solutions for Diverse Challenges
BioNAV™’s capabilities aren’t limited to rare disease patients or even to humans. It can tackle a variety of challenges, including biohazards and conservation.
Domoic acid toxicity has been causing increased incidences of sickness and death in marine wildlife due to climate change. In 2024, thousands of sea lions were reported to have domoic acid toxicity across the California coast. Thus, Unravel has partnered with the Zymo Research Corporation and the Channel Islands Marine & Wildlife Institute (CIMWI) to identify treatments for the sea lions.
In 2025, our partnership has progressed. Using Zymo’s samples and Unravel’s BioNAV™ platform, a drug candidate has been identified. The CIMWI has this molecule in its facilities and is ready for dosing. In 2026, we are hoping to see sea lions rescued by this treatment.
The Unravel Biosciences team extends our gratitude to all of our partners, investors, advocates, advisors, caregivers, clinicians, and more who have supported us this year. We look forward to progressing more in 2026.